A rainy morning can wreck probably the most best coiffure. Hair turns into frizzy, curls fall aside and directly hair turns into hopelessly wavy. It is not accident or the dryer: it is natural chemistry. However what precisely is occurring within every hair that makes water so robust?
Every strand is basically shaped from keratin, a fibrous protein wealthy in sulfur that accumulates, above all, within the outermost layers of the hair: the cuticle and the cortex. Those spaces supply hair with resistance, elasticity and form, and are subsequently maximum delicate to moisture and warmth.
Hair construction. Alfabellezza/Wikimedia Commons
Keratin chains are held in combination through tens of millions of chemical bonds which are grouped into 3 varieties: disulfide, ionic, and hydrogen. Every of them has a distinct serve as within the look and behaviour of the hair.
Hydrogen bonds that act like Velcro
Disulfide bonds, shaped between sulfur atoms (-SS-), are covalent bonds and, subsequently, very robust. They shape the structural pillar of hair fibers. In directly hair, they’re dispensed frivolously, whilst in curly hair, spaces with a better density of those bonds seem, which reasons the fiber to bend or twist.
Disulfide bonds can simplest be damaged through chemical processes, reminiscent of straightening or perm therapies, which use lowering merchandise, reminiscent of ammonium thioglycolate, to damage them down. By way of the usage of them, the fiber may also be simply formed (straightened or twisted) whilst its molecular construction is rearranged. Then, oxidizing brokers, reminiscent of hydrogen peroxide (hydrogen peroxide), stabilize the brand new hair form. By way of editing the inner group of keratin, the result’s long-lasting, even though it reduces its resistance and shine.
To the contrary, hydrogen bonds are a lot weaker, but additionally extra considerable. Already within the 60s, it was once estimated that there are about 9 hydrogen bonds for each disulfide bond in hair fibers. This percentage implies that hydrogen bonds, regardless of their particular person fragility, are liable for brief adjustments within the form of the hair when exterior brokers reminiscent of water or warmth interfere.
In fact, those don’t seem to be chemical bonds within the strict sense, however susceptible electrostatic points of interest between a hydrogen atom (with a partial sure rate) and any other extremely electronegative one, reminiscent of oxygen or nitrogen. In keratin, they act as a “velcro” that holds the strands in combination. Ionic bonds also are in response to these kind of points of interest between definitely and negatively charged protein practical teams.
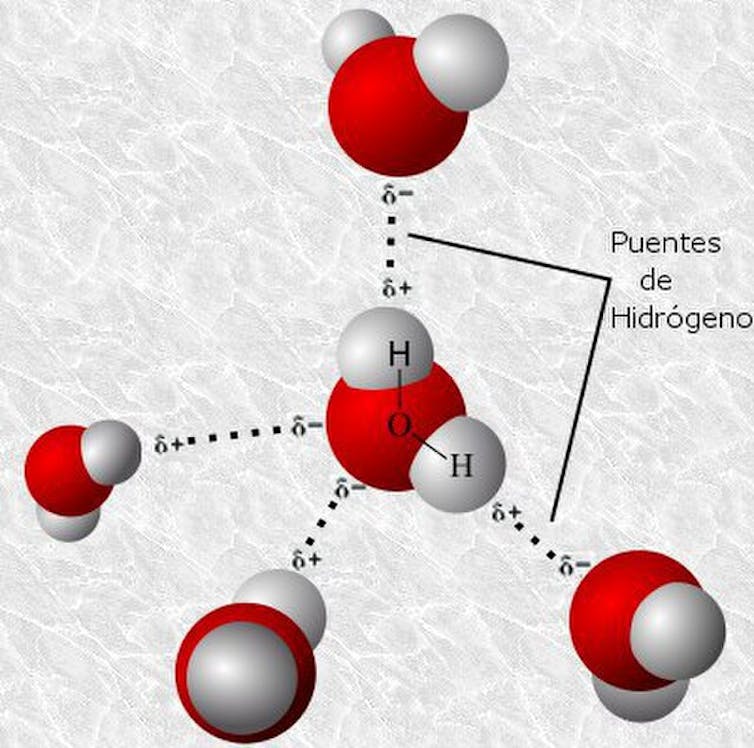
three-D type of water molecules hooked up through hydrogen bonds. Wikimedia Commons Horrible frizziness
When the hair fiber absorbs moisture, the water molecules disrupt the hydrogen bonds of the keratin, competing for a similar issues of appeal and changing them. As those hydrogen “bonds” destroy, rainy hair turns into extra versatile, but additionally vulnerable to deformation.
Those reorganizations on the molecular degree give an explanation for curliness: when the air is full of humidity, water infiltrates between the keratin chains, developing spaces with other quantities of lively bonds, which reasons unequal inner tensions. The result’s fiber that bends irregularly and reasons horrible frizz.
To fight this, anti-frizz merchandise paintings through protecting the hair with a skinny hydrophobic layer (oils, silicones or waxes) that forestalls water from coming into. They do not do away with the issue, however they assist in making the hydrogen bonds extra strong for longer.
Warmth correction
Those hyperlinks will also be intentionally manipulated. The warmth from a blow dryer or flat iron quickly breaks the hydrogen bonds and lets in us to straighten or curl our hair. When it cools or dries, the coiffure is “set”, till moisture intervenes once more. Due to this fact, straightening hair on a wet day is most often an workout in persistence: the water vapor within the air breaks the bridges once more, and the hair returns to its unique form.
The similar is going for curls: water or sweat loosens the waves as a result of moisture prevents the ones bonds. All this explains the pliancy of hair and its skill to bear in mind a coiffure for hours.
This phenomenon, a ways from being simply a classy downside, is an ideal demonstration of ways a bodily exchange so simple as the volume of water vapor within the air can rearrange tens of millions of chemical bonds in an issue of seconds.
When science interprets into hair care
Wisdom of hair chemistry has impressed a brand new era of goods that promise to “renew hair from the inside out.” Some hair therapies (so-called bond developers) attempt to artificially recreate disulfide bonds or offer protection to hydrogen bonds all through competitive processes. Whilst no longer miraculous, they replicate how science interprets into hair care.
Each and every time we comb, straighten, or just let the moisture take its toll, 1000’s of bonds are damaged and rebuilt. So the following time our hair rebels on a wet day, we will blame the elements… or thank chemistry for its murals.






