The colours of rubies and emeralds are so hanging that they outline sun shades of crimson and inexperienced – ruby crimson and emerald inexperienced. However have you ever ever questioned how they get the ones colours?
I’m an inorganic chemist. Researchers in my box paintings to know the chemistry of all of the parts that make up the periodic desk. Many inorganic chemists focal point at the transition metals – the weather in the midst of the periodic desk. The transition metals come with many of the metals you’re accustomed to, like iron (Fe) and gold (Au).
One characteristic of compounds made with transition metals is their intense colour. There are lots of examples in nature, together with gems and paint pigments. Even the colour of blood comes from the protein hemoglobin, which accommodates iron.
Investigating the colours of compounds containing transition metals leads you into some in point of fact superb science – that’s a part of what drew me to review this box.
Rubies and emeralds are nice examples of ways a small quantity of a transition metallic – on this case, chromium – can create an exquisite colour in what would in a different way be a rather boring-looking mineral.
Minerals and crystals
Rubies seem crimson as a result of they soak up blue and inexperienced gentle.
benedek/E+ by the use of Getty Pictures
Each rubies and emeralds are minerals, which is a kind of rock with a constant chemical composition and a extremely ordered construction on the atomic stage.
When this extremely ordered construction extends in all 3 dimensions, the mineral turns into a crystal.
With a idea evolved by way of physicists within the Twenties known as crystal box idea, scientists can give an explanation for why rubies and emeralds have the colours they do. Crystal box idea makes predictions about how a transition metallic ion’s construction is suffering from the opposite atoms surrounding it.
Rubies are basically made up of the mineral corundum, which consists of the weather aluminum and oxygen in a typical, repeating array. Every aluminum ion is surrounded by way of six oxygen ions.
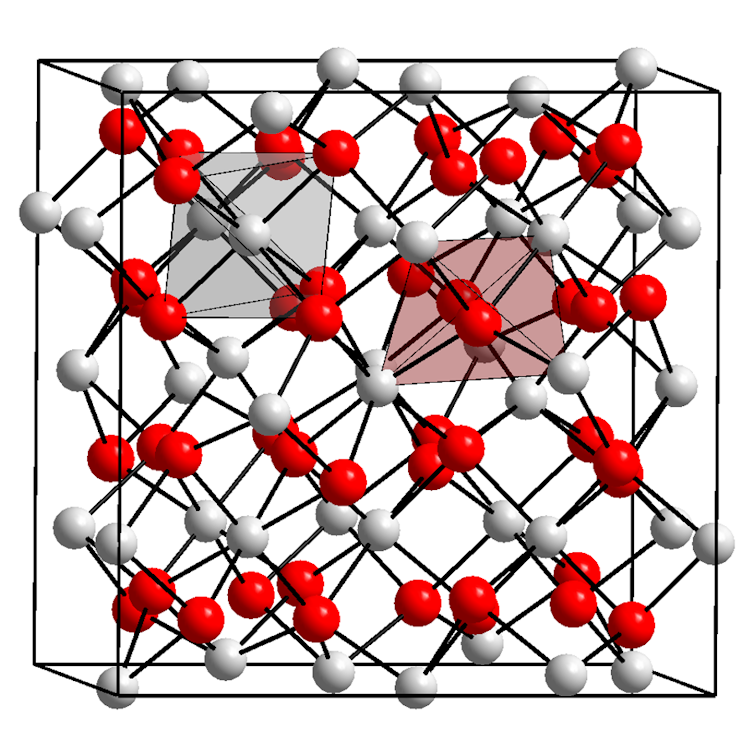
A crystal of corundum seems like this on the atomic stage, with the aluminum ions proven as crimson balls and the oxygen ions proven as white balls. Every aluminum ion is surrounded by way of six oxygen ions, and each and every oxygen by way of 4 aluminums.
Eigenes Werk/Wikimedia Commons, CC BY-SA
Emeralds are basically made up of the mineral beryl, which is constituted of the weather beryllium, aluminum, silicon and oxygen. Beryl’s crystal construction is extra sophisticated than corundum’s as a result of the extra parts within the method, however each and every aluminum ion is once more surrounded by way of six oxygen ions.

Emeralds seem inexperienced as a result of they soak up crimson and blue gentle.
SunChan/E+ by the use of Getty Pictures
Natural corundum and beryl are colorless. The bright colours of rubies and emeralds come from the presence of very small quantities of chromium. The chromium replaces about 1% of the aluminum within the corundum or beryl crystal when a ruby or emerald paperwork underground at a prime temperature and power.
However how can one component – chromium – create the crimson colour of a ruby and inexperienced colour of an emerald?
Colour science
Rubies and emeralds have the colours they do as a result of, like many ingredients, they soak up some colours of sunshine. Maximum visual gentle, like daylight, consists of all of the colours of the rainbow: crimson, orange, yellow, inexperienced, blue, indigo and violet. Those colours make up the visual gentle spectrum, which is simple to bear in mind as ROY G BIV.
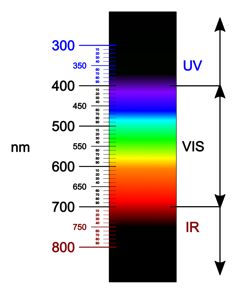
Items soak up some visual gentle wavelengths and mirror others, which is why we see them as having a colour.
Fulvio314/Wikimedia Commons, CC BY-SA
One of the crucial primary the reason why items have a colour is as a result of they soak up a number of of those visual colours of sunshine. If a substance absorbs, as an example, crimson gentle, it signifies that the crimson gentle will get trapped within the substance and the opposite colours mirror again on your eyes. The colour you spot is the sum of the remainder gentle, which might be within the green-to-blue vary. If a substance absorbs blue, it is going to glance crimson or orange to you.
In contrast to the colorless aluminum ion, the chromium ion absorbs blue and inexperienced gentle when surrounded by way of the oxygen ions. The crimson gentle is mirrored again, in order that’s what you spot in rubies.
In an emerald, even if the chromium is surrounded by way of six oxygen ions, there’s a weaker interplay between the chromium and the encompassing oxygen ions. That’s because of the presence of silicon and beryllium within the beryl crystal. They reason the emerald to soak up blue and crimson gentle, leaving the fairway so that you can see.
The facility to track the homes of transition metals like chromium via converting what’s surrounding this can be a core technique in my box of inorganic chemistry. Doing so can assist scientists perceive the fundamental science of metal-containing compounds and the design of chemical substances for particular functions.
You’ll be able to take satisfaction within the superb colours of the gems, however via chemistry, you’ll be able to additionally see how nature creates the ones colours the usage of an never-ending number of complicated buildings made with the weather within the periodic desk.





