“What is radium and why is it dangerous?” – Aurora, 10, Laredo, Texas
The component radium can also be present in extraordinarily tiny quantities within the Earth’s crust and oceans, and in its natural shape this is a comfortable silvery steel. To an untrained eye, a small piece of radium would possibly seem like a chip off a standard grey rock. However radium can invisibly emit radiation – calories and small fragments of itself – that you’ll be able to’t really feel, see or odor. And that invisible radiation can harm you, with out you even noticing straight away.
What’s happening with this silent risk that may stealthily harm your frame in tactics that may take years to expose themselves?
As a chemist, I’m fascinated with what makes other parts secure to deal with or hazardous. This bad unlock of radiation is known as radioactivity, and despite the fact that its supply would possibly glance unassuming, it will possibly burn you and even come up with sicknesses that don’t manifest for years.
Atoms and isotopes
The whole lot you notice round you – your pores and skin, rocks, the pages of books – is all made up of various mixtures of extraordinarily small debris known as atoms.
An atom has a small, dense middle known as the nucleus. Negatively charged debris known as electrons transfer across the nucleus. Throughout the nucleus, there are two varieties of debris: undoubtedly charged protons and impartial neutrons.
All atoms with the similar choice of protons of their nuclei are the similar component. But even so radium, some parts you’ll have heard of include carbon and oxygen. All carbon atoms have six protons and all oxygen atoms have 8 protons. Radium atoms are a lot heavier – all radium atoms have 88 protons.
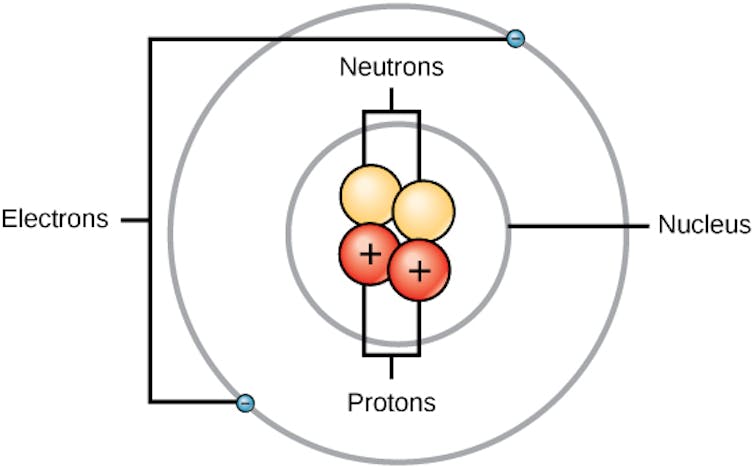
A simplified type of an atom, the place the nucleus, containing neutrons and definitely charged protons, sits within the middle surrounded by means of negatively charged electrons.
CNX OpenStax/Wikimedia Commons, CC BY
Curiously, it’s imaginable for atoms of the similar component to have other numbers of neutrons. Atoms of the similar component with other numbers of neutrons are known as isotopes. As an example, two carbon atoms would every have six protons, however one may have six neutrons whilst any other will have seven or 8.
The choice of protons and neutrons packed in combination within the nucleus determines whether or not the nucleus of an isotope is solid or no longer. If the nucleus isn’t solid, issues can stand up.
Radioactive decay
The nucleus of every atom desires to be solid, however simplest sure preparations of protons and neutrons make that imaginable. The choice of protons and neutrons wouldn’t have to be equivalent, however some mixtures make for a cheerful, or solid, coexistence within the nucleus whilst others don’t.
A nucleus with an unsatisfied mixture of protons and neutrons may destroy down or become worse someway. That procedure is known as radioactivity or radioactive decay.
Parts are radioactive in the event that they decay by means of liberating portions of the nucleus or high-energy debris.
Armtuk/Wikimedia Commons, CC BY
That radioactive decay procedure releases some type of radiation from the nucleus. This radiation can take the type of tiny debris transferring abruptly or high-energy electromagnetic waves rising from the nucleus. It’s that radiation – the high-energy debris and waves taking pictures out from the nucleus of risky atomic nuclei – that may make you in poor health.
There are several types of radioactive decay. In a single case, an atom decays by means of kicking out a small fragment of itself this is made up of 2 protons and two neutrons. For the reason that choice of protons determines what component we’ve, decay that adjustments the choice of protons in an atom turns it into a special component.
Radioactive decay can also be reasonably gradual, even though. It will possibly take hundreds of years for one component to decay into a special one.
The case of radium
All radium atoms are risky and radioactive. Many of those isotopes decay in no time, however Ra-226, which has 138 neutrons and 88 protons and is the most typical, decays the slowest. It takes 1,600 years for part a pattern of Ra-226 to decay.
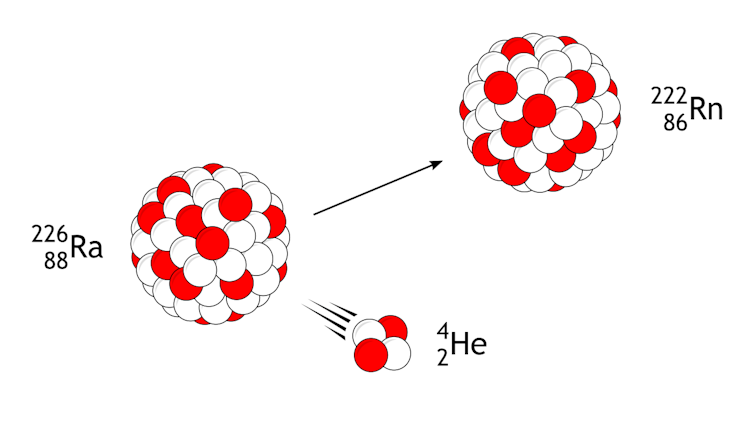
Radium undergoes alpha decay, the place it loses a fraction of its nucleus containing two protons and two neutrons, and then it turns into radon.
MikeRun/Wikimedia Commons, CC BY-SA
As Ra-226 decays, it loses two protons and two neutrons, which turns it into an isotope of radon. Then the radon decays, and the atom ultimately reaches a solid shape because the component lead. Each and every step in that decay collection releases extra nuclear radiation.
Another parts in nature without a solid isotope are technetium, polonium, actinium and uranium.
Results at the human frame
The nuclear radiation emitted when radium and different parts decay can harm the cells within the human frame. It can result in cancers or different well being issues.

Marie and Pierre Curie experimented with radium, which ended up inflicting well being headaches for them.
André Castaigne
Whether or not you’re uncovered to numerous radiation briefly, like making the error of strolling round for a couple of hours with radioactive subject material on your pocket, otherwise you’re uncovered to just a bit over a very long time, the high-energy debris and electromagnetic waves from nuclear radiation can result in critical well being issues, together with burns and cancers.
Remarkably, despite the fact that radioactivity is a risk to lifestyles, scientists can regulate and use it to diagnose and deal with sicknesses – together with cancers. If the radiation is delivered exactly to the place most cancers cells are, the radiation can ruin the ones rogue cells wreaking havoc within the frame.
Individuals who paintings professionally with radioactive fabrics wish to apply strict pointers and procedures to offer protection to themselves. They use particular shields and radiation detectors, they usually reduce the period of time they’re uncovered to any radioactivity.
Pierre and Marie Curie, who found out radium in 1898, suffered one of the side effects of radioactivity. Pierre skilled radioactive burns, and Marie died from a blood illness most probably led to by means of power radiation publicity. Over 100 years later, her notebooks are nonetheless radioactive.
And because interest has no age prohibit – adults, tell us what you’re questioning, too. We gained’t have the ability to solution each and every query, however we will be able to do our very best.






